Oxygen has 8 electrons. Oxygen, a chemical element with the symbol O and atomic number 8, contains 8 electrons in its atomic structure.
It is a highly reactive element and is essential for various biological processes, as well as being a key component of Earth’s atmosphere. Oxygen is known for its role in supporting combustion and respiration, and it is the third most abundant element in the universe.
Its electron configuration includes 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and 4 electrons in the 2p orbital. Understanding the electron configuration of oxygen is crucial in comprehending its chemical properties and interactions with other elements.
Credit: socratic.org
The Atomic Structure Of Oxygen
Oxygen is a chemical element with the symbol O and atomic number 8. Understanding its atomic structure sheds light on its fundamental properties.
Atomic Number Significance
The atomic number of oxygen, which is 8, represents the number of protons in its nucleus.
This defines its chemical behavior and its place in the periodic table.
Electron Shells And Configuration
Oxygen has 2 electron shells, with 6 electrons in the outer shell and 2 in the inner shell.
The electron configuration of oxygen is 1s2 2s2 2p4.
Peering Into The Periodic Table
The periodic table is an essential tool for chemists and scientists alike. It provides a systematic arrangement of chemical elements based on their atomic structure, properties, and electron count. By peering into the periodic table, scientists can predict how elements will interact with one another and understand the fundamental nature of matter.
Position And Properties
Oxygen, with the symbol O, is a non-metallic element found in group 16, period 2 of the periodic table. It has an atomic number of 8, which means it has eight protons and eight electrons. Oxygen is the third most abundant element in the universe, after hydrogen and helium, and is essential for life as we know it.
Oxygen has a number of unique properties that make it crucial for living organisms. It is a highly reactive element that readily forms compounds with other elements, such as hydrogen to form water. Oxygen is also essential for respiration, allowing living organisms to extract energy from food.
Periodic Trends And Electron Count
Periodic trends are patterns that emerge when elements are arranged in the periodic table. One such trend is electron count. Elements in the same group have the same number of valence electrons, which are the electrons in the outermost shell of an atom. Oxygen, being in group 16, has six valence electrons.
Electron count is an important factor in determining an element’s reactivity. Elements with a full outer shell, like the noble gases, are generally unreactive, while elements with a partially filled outer shell, like oxygen, are more reactive and tend to form compounds with other elements.
In conclusion, oxygen has eight electrons, six of which are valence electrons. Its position in the periodic table and electron count contribute to its unique properties and essential role in the natural world.
Electron Count Essentials
Understanding the electron count of oxygen is crucial. Let’s delve into Electron Count Essentials.
Valence Electrons And Bonding
Oxygen has 6 valence electrons, involved in bonding.
Inner Electrons And Stability
Inner electrons in oxygen contribute to its stability.
Chemical Bonding And Oxygen
How Many Electrons Does Oxygen Have? Oxygen has 8 electrons, with 6 in its outer shell, making it capable of forming 2 bonds. Chemical bonding helps oxygen share electrons with other elements, creating stable compounds crucial for life.
Covalent Bonds In Molecules
Covalent bonds play a crucial role in the formation of molecules, and oxygen is no exception. Oxygen, with its atomic number of 8, possesses 8 electrons. These electrons are organized into different energy levels or shells, with 2 electrons in the first shell and 6 in the second shell.
Oxygen’s Role In Compound Formation
Oxygen’s electron configuration makes it highly reactive, allowing it to readily participate in compound formation. It tends to form covalent bonds, in which electrons are shared between atoms to achieve stability.
Oxygen has a strong affinity for electrons, and its electronegativity is relatively high. This means that in a covalent bond, oxygen attracts the shared electrons more strongly, giving it a slightly negative charge.
When oxygen combines with other elements, it can form various compounds. For example, when oxygen bonds with hydrogen, it forms water (H2O), a vital compound for all living organisms. In this case, oxygen shares electrons with two hydrogen atoms, creating a stable molecule.
Oxygen also participates in compound formation through double and triple bonds. These bonds occur when oxygen shares more than one pair of electrons with another atom, resulting in the formation of molecules such as carbon dioxide (CO2) and ozone (O3).
In carbon dioxide, each oxygen atom shares two pairs of electrons with a carbon atom, forming a double bond. This compound is essential for photosynthesis and is a byproduct of various combustion processes.
Ozone, on the other hand, consists of three oxygen atoms bonded together through double bonds. It plays a crucial role in the Earth’s atmosphere, absorbing harmful ultraviolet radiation from the Sun.
In summary, oxygen’s electron configuration and electronegativity make it a key player in compound formation. Its ability to form covalent bonds allows it to participate in various chemical reactions and contribute to the formation of essential compounds.
Oxygen Ions: Gaining And Losing Electrons
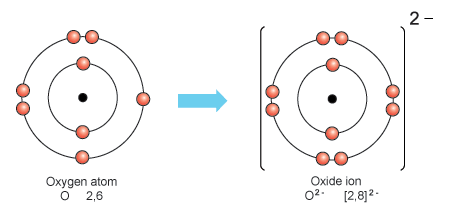
Oxygen ions, also known as oxide ions, play a crucial role in chemical reactions and the formation of compounds. Understanding how oxygen gains and loses electrons to become ions is essential in comprehending its reactivity and behavior in various chemical processes.
Formation Of Oxide Ions
Oxygen, with its atomic number 8, typically forms oxide ions by gaining two electrons. This process occurs when oxygen atoms react with other elements to achieve a stable electronic configuration. The gain of electrons allows oxygen to achieve a full outer shell of eight electrons, resembling the configuration of noble gases.
Electron Transfer In Ionic Bonds
When forming ionic bonds, oxygen can gain or lose electrons to achieve a stable electronic configuration. In the case of gaining electrons, oxygen forms negatively charged ions, known as oxide ions. On the other hand, when oxygen loses electrons, it forms positively charged ions.
Credit: www.quora.com
Quantum Mechanics And Electron Arrangement
Oxygen has 8 electrons arranged in 2 in the first shell and 6 in the second shell according to Quantum Mechanics.
Quantum Numbers Explained
In the realm of quantum mechanics, the behavior of electrons is described by a set of unique identifiers known as quantum numbers. These numbers provide crucial information about an electron’s energy, orbital shape, and orientation within an atom. By understanding these quantum numbers, we can unravel the intricate electron arrangement in oxygen and other elements.
Electron Orbitals And Configurations
Electron orbitals are regions within an atom where electrons are likely to be found. Each orbital can hold a specific number of electrons, determined by the quantum numbers. Oxygen, with its atomic number of 8, has eight electrons. Let’s explore how these electrons are arranged in the different orbitals. When it comes to oxygen’s electron configuration, it follows the pattern 1s^2, 2s^2, 2p^4. This notation represents the distribution of electrons in the atom’s energy levels and sublevels.
The first number signifies the principal quantum number, indicating the energy level. The letter denotes the sublevel, while the exponent specifies the number of electrons occupying that sublevel. In the case of oxygen, the first two electrons occupy the 1s orbital, followed by two more in the 2s orbital. The remaining four electrons are distributed across the 2p orbitals. The 2p orbitals consist of three separate orbitals: 2p_x, 2p_y, and 2p_z. Each of these orbitals can hold a maximum of two electrons, resulting in a total of six electrons in the 2p sublevel.
To summarize, oxygen has a total of eight electrons, with two in the 1s orbital, two in the 2s orbital, and four in the 2p orbitals. This arrangement follows the principles of quantum mechanics, providing insights into the electron distribution and behavior within the atom.
In conclusion, understanding the quantum mechanics behind electron arrangement allows us to comprehend the intricate details of elements like oxygen. By deciphering the electron configurations and orbital arrangements, scientists can unlock a wealth of knowledge about an atom’s properties and its interactions with other elements. This knowledge forms the foundation of many scientific disciplines and contributes to our understanding of the world around us.
Experimental Methods To Determine Electron Count
Experimental methods, such as spectroscopy and electron paramagnetic resonance, are used to determine the electron count of oxygen. These techniques provide valuable insights into the number of electrons present in oxygen atoms, aiding in the understanding of its chemical properties.
Spectroscopy And Its Revelations
Oxygen’s electron count is determined through spectroscopy methods.
X-ray Crystallography Insights
X-ray crystallography provides detailed insights into electron arrangement.
Practical Implications Of Oxygen’s Electrons
Discover the practical implications of oxygen’s electrons and its significance in various aspects.
Importance In Biological Systems
Oxygen’s electrons are vital for energy production in living organisms.
- Oxygen is crucial for cellular respiration.
- Plays a role in the electron transport chain.
- Facilitates the creation of ATP.
Industrial Applications Of Oxygen
Oxygen’s electrons are utilized in numerous industrial processes.
- Welding: Oxygen supports combustion for welding processes.
- Steel production: Essential for the oxidation of impurities in steelmaking.
- Medical: Used in hospitals for respiratory therapies.
Frequently Asked Questions
How Many Electrons Does Oxygen Have?
Oxygen has 8 electrons, with 2 in its inner shell and 6 in its outer shell. This configuration makes it highly reactive and essential for various chemical processes.
What Is The Significance Of Oxygen’s Electron Configuration?
Oxygen’s electron configuration allows it to readily form bonds with other elements, playing a crucial role in the formation of water and many organic compounds essential for life.
How Do Oxygen’s Electrons Contribute To Its Properties?
The arrangement of electrons in oxygen influences its reactivity, making it vital for respiration, combustion, and various industrial processes. Its electron configuration is fundamental to its role in sustaining life on Earth.
Conclusion
Oxygen has eight electrons, two of which are valence electrons. These valence electrons make oxygen highly reactive and able to form bonds with other atoms. Understanding the electron configuration of oxygen is crucial in many fields, including biology, chemistry, and physics.
By learning about the electron structure of oxygen, we can better understand the properties and behaviors of this essential element.