Dry ice is approximately minus 109.3 degrees Fahrenheit or minus 78.5 degrees Celsius. Dry ice, which is solid carbon dioxide, is extremely cold and can cause frostbite on contact with skin.
Dry ice is a fascinating and versatile substance that has a wide range of applications, from creating spooky special effects to preserving perishable items during shipping. Understanding its properties and best practices for handling is essential for safety and effective use.
In this blog, we will explore the characteristics of dry ice, its uses, safety considerations, and environmental impact. Whether you are a science enthusiast, a business owner, or simply curious about this unique form of ice, this guide will provide you with valuable insights into the world of dry ice.

Credit: www.cganet.com
The Essence Of Dry Ice
Dry ice is extremely cold, with a temperature of -109. 3°F (-78. 5°C), making it ideal for various applications like preserving perishable items and creating spooky special effects. Its frigid nature stems from being solid carbon dioxide, a unique substance that sublimates directly from solid to gas.
Dry ice is a fascinating substance that can be used for a variety of purposes. Its unique chemical composition and physical properties make it a popular choice for cooling and preserving items. The most significant aspect of dry ice is its ability to maintain extremely low temperatures. In this section, we will take a closer look at the chemical composition and physical properties of dry ice.
Chemical Composition
Dry ice is the solid form of carbon dioxide (CO2), which is a colorless, odorless gas that makes up only 0.04% of the Earth’s atmosphere. Carbon dioxide is produced during respiration, fermentation, and combustion processes. It is also a byproduct of many industrial processes, including the production of cement, steel, and ammonia. To create dry ice, liquid carbon dioxide is pressurized and cooled to a temperature of around -109.3°F (-78.5°C). At this temperature and pressure, the carbon dioxide turns into a solid without going through a liquid phase. The resulting dry ice is then cut into blocks or pellets for commercial use.
Physical Properties
Dry ice is a unique substance with several physical properties that make it useful for a variety of applications. Some of these properties include:
- Extremely low temperature: As mentioned earlier, dry ice is incredibly cold, with a temperature of around -109.3°F (-78.5°C). This makes it an excellent choice for freezing and preserving items.
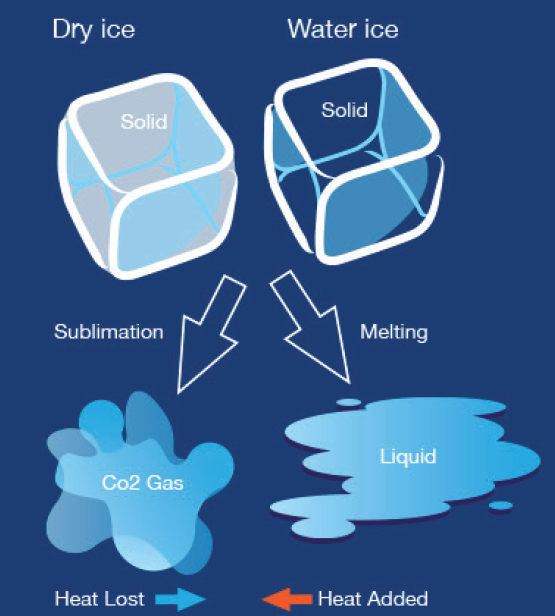
- Sublimation: Dry ice does not melt like regular ice. Instead, it sublimates, which means it turns directly from a solid to a gas without going through a liquid phase. This makes it an ideal choice for certain cleaning applications, such as removing graffiti or cleaning electrical components.
- Density: Dry ice is denser than regular ice, which means it can cool items more effectively. This makes it a popular choice for shipping and transporting perishable goods.
- Non-toxic: Dry ice is non-toxic, which means it is safe to use in food and medical applications.
In conclusion, dry ice is a fascinating substance with unique properties that make it a popular choice for a variety of applications. Its ability to maintain extremely low temperatures, its sublimation properties, and its density make it an ideal choice for cooling, preserving, and transporting items.
Credit: www.gulfcryo.com
Comparing Temperatures
When it comes to temperatures, dry ice is significantly colder than water ice. Let’s explore the differences:
Dry Ice Vs. Water Ice
How Cold is Dry Ice? Dry ice is approximately -109.3°F (-78.5°C), while water ice maintains a temperature of 32°F (0°C).
Industrial And Domestic Freezing Points
| Substance | Freezing Point |
|---|---|
| Dry Ice | -109.3°F (-78.5°C) |
| Water Ice | 32°F (0°C) |
Production Of Dry Ice
Dry ice is a fascinating substance that is commonly used for various purposes, such as preserving perishable items, creating special effects, and even cleaning. But have you ever wondered how this icy wonder is produced? In this section, we will delve into the manufacturing process of dry ice and the safety measures involved in handling it.
Manufacturing Process
The production of dry ice involves a unique process that starts with pressurizing and cooling carbon dioxide gas. Here’s a step-by-step breakdown of the manufacturing process:
- Carbon Dioxide Collection: Carbon dioxide gas is typically collected as a byproduct of various industrial processes or extracted from the atmosphere.
- Purification: The collected carbon dioxide gas undergoes purification to remove impurities and contaminants.
- Compression: The purified carbon dioxide gas is then compressed to increase its pressure.
- Refrigeration: The compressed carbon dioxide gas is cooled, causing it to undergo a phase change from a gas to a solid, known as deposition.
- Extrusion: The solid carbon dioxide, also known as dry ice, is extruded through a die, which gives it the desired shape, such as pellets, blocks, or slices.
- Packaging: Finally, the produced dry ice is packaged and ready for transportation and use.
Safety Measures In Handling
While dry ice has numerous applications, it’s crucial to handle it with utmost care due to its extremely cold temperature. Here are some safety measures to keep in mind when handling dry ice:
- Protective Gear: Always wear insulated gloves or use tongs to handle dry ice, as direct contact with skin can cause frostbite.
- Ventilation: Ensure that the area where dry ice is stored or used is well-ventilated to prevent the buildup of carbon dioxide gas, which can displace oxygen.
- Storage: Store dry ice in a well-insulated container or cooler with proper ventilation to prevent excessive sublimation.
- Transportation: When transporting dry ice, ensure proper packaging and ventilation to avoid the risk of carbon dioxide buildup in enclosed spaces.
- Usage: Never ingest or consume dry ice, as it can cause severe internal injuries.
- Disposal: Allow any unused dry ice to sublimate in a well-ventilated area or consult local guidelines for proper disposal methods.
By following these safety measures, you can handle dry ice effectively and minimize the associated risks.

Credit: www.quora.com
Thermal Dynamics
Dry ice is extremely cold, reaching temperatures of around -109. 3°F (-78. 5°C). It sublimates directly from solid to gas, making it a unique cooling agent in thermal dynamics. Its frosty properties make it ideal for various applications like preserving perishable items during shipping.
Thermal Dynamics When it comes to understanding dry ice, it’s essential to grasp the concept of thermal dynamics. Simply put, thermal dynamics is the study of the movement of heat and its conversion to other forms of energy. In the case of dry ice, thermal dynamics play a crucial role in determining its temperature and behavior. In this section, we’ll explore how heat transfer and sublimation explain the coldness of dry ice. Heat Transfer Heat transfer is the process by which heat energy is moved from one object to another.
In the case of dry ice, heat transfer occurs when it’s placed in contact with a warmer object. The heat energy flows from the warmer object to the dry ice, causing it to absorb the heat and increase in temperature. However, since dry ice is so cold (-109.3°F or -78.5°C), it doesn’t take long for it to absorb all the heat energy from the warmer object and bring it down to its own temperature. Sublimation Explained Sublimation is the process by which a solid substance transitions directly into a gas without going through the liquid phase. This is precisely what happens with dry ice when it’s exposed to room temperature.
Since dry ice is so cold, it absorbs heat energy from its surroundings, causing it to undergo sublimation. This means that it transitions from a solid to a gas without melting first. The sublimation process is what creates the iconic fog-like effect that we often associate with dry ice. In summary, the thermal dynamics of dry ice are what make it so unique and interesting. Its incredibly low temperature allows it to absorb heat energy quickly, making it an excellent tool for refrigeration and transportation of perishable goods.
Its ability to undergo sublimation also makes it a popular choice for special effects, such as creating fog for a spooky Halloween ambiance. Understanding the science behind dry ice can help us appreciate its many uses and applications.
Applications Of Dry Ice
Dry ice, the solid form of carbon dioxide, is widely used across various industries due to its unique properties and applications. From commercial uses to scientific research, dry ice plays a crucial role in a multitude of applications.
Commercial Uses
Dry ice is commonly utilized in the commercial sector for various applications such as:
- Shipping and transportation of perishable goods
- Food and beverage industry for freezing and preserving products
- Cleaning and blasting applications in industrial settings
Scientific Research
In the realm of scientific research, dry ice finds its applications in:
- Creating controlled environments for scientific experiments
- Preservation of biological samples and specimens
- Simulating extreme environmental conditions for testing purposes
Safety Precautions
When working with dry ice, it is essential to prioritize safety to prevent any accidents or injuries. By following the necessary precautions, you can handle dry ice effectively and minimize potential risks.
Personal Protective Equipment
Wearing appropriate personal protective equipment (PPE) is crucial to ensure your safety when handling dry ice. The following PPE should be worn:
- Thick insulated gloves to protect your hands from direct contact with dry ice.
- Safety goggles or a face shield to shield your eyes and face from any potential splashes or flying debris.
- Long-sleeved clothing and pants to cover your skin and reduce the risk of exposure.
- Closed-toe shoes or boots to protect your feet from accidental contact.
Remember, PPE should be worn at all times when handling dry ice, regardless of the quantity or duration of exposure.
First Aid For Dry Ice Burns
Despite taking precautions, accidents can still happen. In the event of a dry ice burn, immediate first aid is crucial to minimize further damage. Here are the steps to follow:
- Move away from the source of the burn and find a safe area.
- Remove any clothing or jewelry that may be in contact with the affected area.
- Rinse the burn with cool water for at least 10 to 15 minutes to help reduce the temperature.
- Cover the burn with a sterile, non-stick bandage or cloth to protect it from further contamination.
- Seek medical attention if the burn is severe or covers a large area of the body.
Remember, dry ice burns can be more severe than regular burns due to its extremely cold temperature. Prompt and proper first aid is essential for a speedy recovery.
Storing Dry Ice
Dry ice is extremely cold, reaching temperatures as low as -78. 5°C (-109. 3°F). When storing dry ice, it is important to handle it with care and keep it in a well-ventilated container to prevent the buildup of carbon dioxide gas.
Optimal Storage Conditions
Store dry ice in a well-insulated container. Ensure the container has ventilation holes. Avoid storing in airtight containers.
Maximizing Shelf Life
Wrap dry ice in newspaper or towels. Place it in the coldest part of the freezer. Use dry ice within 24 hours of purchase.
Environmental Impact
Considering the ecological impact of dry ice, it is crucial to understand its environmental implications and how it affects our planet.
Ecological Considerations
Dry ice is carbon dioxide in solid form, which means that when it sublimates, it directly transforms into gas without any liquid stage.
Disposal Guidelines
- Always allow dry ice to sublimate in a well-ventilated area to prevent any potential harm to the environment.
- Do not dispose of dry ice in regular trash bins or down the drain as it can have detrimental effects.
- Consider reusing dry ice whenever possible to minimize waste and environmental impact.
Frequently Asked Questions
How Cold Is Dry Ice?
Dry ice is extremely cold, with a temperature of -78. 5 degrees Celsius (-109. 3 degrees Fahrenheit). Its low temperature makes it ideal for various applications, such as freezing food, creating smoke effects in entertainment, and preserving medical supplies.
How Is Dry Ice Made?
Dry ice is made by pressurizing and cooling carbon dioxide gas until it turns into a solid. The process involves compressing the gas and removing the heat, causing it to transform directly into a solid without going through a liquid state.
This solid carbon dioxide is then broken into small pellets or blocks, which we commonly refer to as dry ice.
Can You Touch Dry Ice?
It is not advisable to touch dry ice directly with your bare hands. Dry ice is so cold that it can cause frostbite or burn your skin. Always handle dry ice with thick gloves or use tongs when necessary. Remember to use caution and handle it safely to avoid any injuries.
How Long Does Dry Ice Last?
The duration of dry ice depends on various factors, such as the amount of dry ice, the size of the insulated container, and the surrounding temperature. Generally, dry ice can last anywhere from 12 to 24 hours. It gradually turns into gas as it sublimates, so it’s important to use it as soon as possible or store it properly in a well-insulated container.
Conclusion
Dry ice is extremely cold, reaching temperatures of -109. 3°F (-78. 5°C). It is commonly used for cooling and freezing purposes in various industries. Understanding its properties and safe handling is crucial. With proper precautions, dry ice can be a versatile and effective tool for many applications.